
Allevi Blog
How to Choose a Bioink for Use in 3D Biofabrication
- Updated on October 27, 2023
We’ve talked about what bioprinting entails in general. We’ve touched on the types of cells that we can incorporate into bioprinted constructs. We’ve also discussed the potential of this technology to create novel chimeric structures and compared 3D bioprinting to plastic 3D printing. It’s now time to talk about another critical component in all of this: how to choose a bioink (biomaterial formulation) that will provide the scaffolding for the cells of our engineered constructs.
A recent paper titled, “Engineering Hydrogels for Biofabrication”, published in Advanced Materials in 2013 did an outstanding job discussing the state-of-the-art in the field of biomaterials. In this post, we attempt to summarize their work in the context of our own efforts at Allevi.
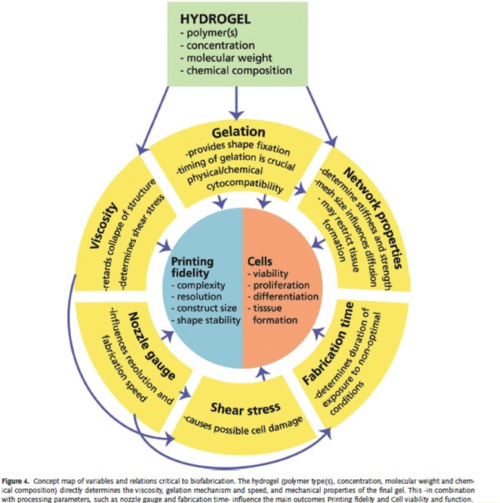
Malda et al. 25th Anniversary Article: Engineering Hydrogels for Biofabrication. Adv. Mater. 2013, 25, 5011–5028
Biomaterials form the scaffolding which supports cells and growth factors during their delivery to damaged tissues and organs. As mentioned in previous pieces and in what will be the subject of a piece coming out later in the week, the 3D environment of the cells is vital in helping maintain cell morphology, differentiation state, and activity. Unfortunately, the 3D scaffolds that have been used in most existing work offer limited spatial control of the cells, ECM, and growth factors encapsulated within them. This is where the additive manufacturing technique of 3D bioprinting comes in to offer the potential of much greater spatial regulation and the ability to design more physiologically accurate tissues. That said, we are still limited in the resolution we can achieve with 3D printing techniques – something which we, at Allevi, are striving to change.
Some factors must be considered when attempting to increase the resolution, i.e. the minimum distinguishable structure size, we can achieve: the viscosity of the solution being printed, the size of the nozzle of the printer head, the speed at which the solution is dispensed, and how the solution undergoes the change from liquid to gel state.
Cells experience shear stress as they are ejected through the nozzle and work remains to be done to determine the maximum shear stress cells can withstand while remaining viable. This will help us determine how small the size of the nozzle can be through which cells are being extruded. Shear-thinning is an interesting material property of some hydrogels in which the shear causes the polymer chains to elongate and reorganize into a less entangled conformation, thereby reducing the viscosity of the gel as the shear is applied. In essence, the more the shear, the more easily the gel can flow.
An important consideration is how we can crosslink gels to form stable structures that retain their 3D conformation. Harsh chemical treatments and heat are not feasible when considering gels encapsulating cells since cells are unable to tolerate these conditions. Increasingly, the focus has been on using ionic crosslinking and photocrosslinking to promote gelation. These techniques will be featured in future posts.
So having considered some of the parameters important in choosing a biomaterial, let’s discuss some commonly used bioinks. There has been a tendency to favor naturally derived polymers such as alginate, gelatin, chitosan, collagen, fibrin, and hyaluronic acid. These polymers have bioactive signals which allow for improved cell viability and proliferation potential. However, the biggest challenge remains the variability between different batches of naturally derived polymers, making it difficult to control experiments with them. This has led to the use of synthetic polymers such as poly(ethylene) glycol (PEG), which allow the creation of highly precise, stable 3D structures but offer lower cell viability due to the absence of bioactive signals. Such signals (peptide sequences and growth factors) must be incorporated into these networks to try and recapitulate the characteristics of natural polymers.
Combinations of synthetic polymers, such as PEGDA, with rigid fibers such as those of polycaprolactone (PCL) may allow for improved structural stability of the constructs. An important point to note is that the degradation process of these two different polymers must be finely tuned so that the hydrogel construct dissolves away as cells start depositing ECM and proteases and other enzymes degrade the gel components, while the fibers retain their position for longer to continue offering the mechanical support to the cells.
So what are some of the challenges we face when it comes to picking the right bioink? Well, we have yet to design one which mimics the ECM closely enough. We need to balance the need for structural stability with the requirement for cells and signals to communicate with each other via diffusion and other transport mechanisms through this matrix. We also need to be able to reproducibly manufacture and assess these constructs (could a 3D bioprinter be the answer to this?) and design a range of materials matching the enormous diversity of scaffolds that support the many different cell types in our body.
Pretty simple, right?

