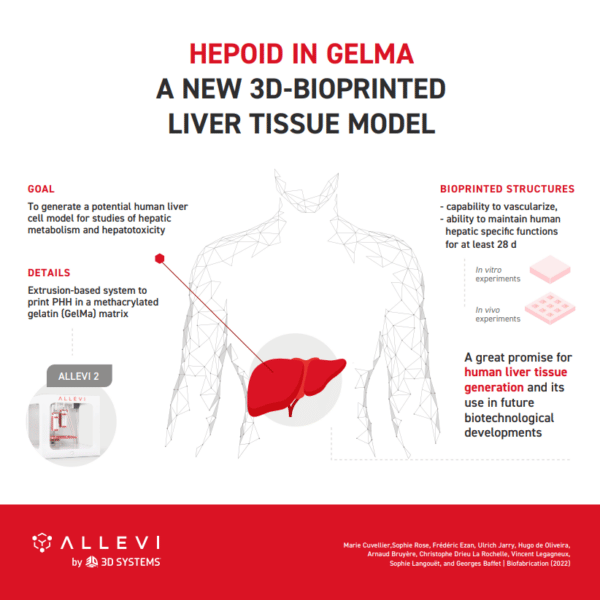
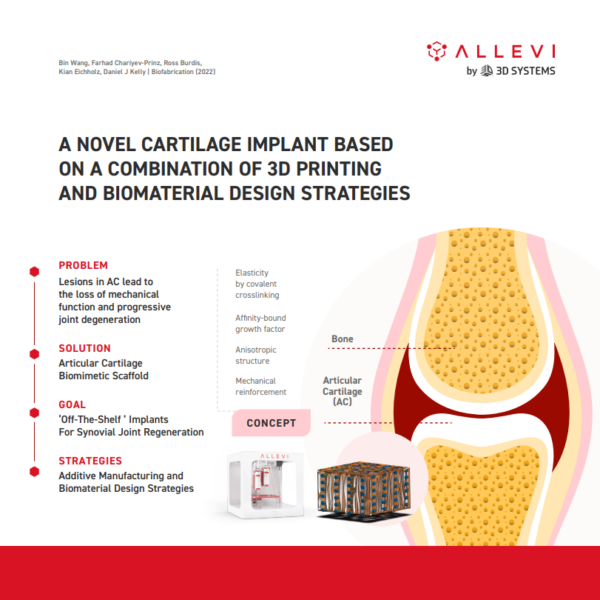
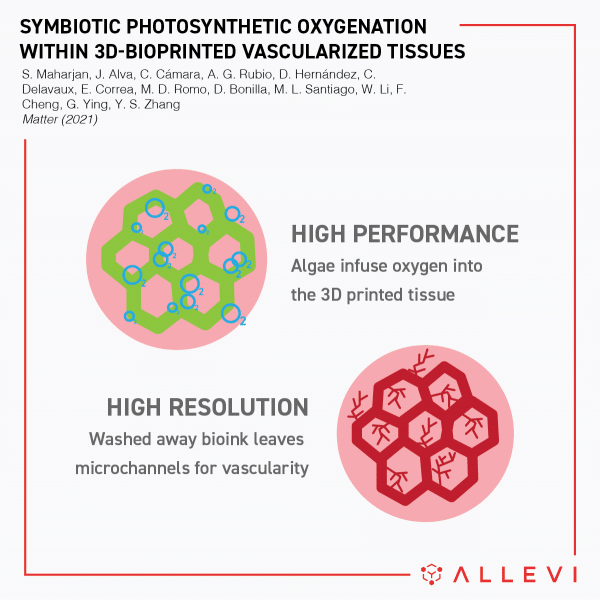
Allevi Announcement: Discontinued Products
We deeply value your interest in Allevi and want to provide information about the changes within our organization. After careful consideration and evaluation, we have made the strategic decision to discontinue production of our Allevi bioprinters, effective July 27, 2023.